Circular Economy and Me – Issue 7

Investigation on Green Catalysts for a Sustainable Transformation from Linear to Circular Chemical Economy by Dr. Mazharul M Islam.
The inventions during all industrial revolutions have been the most powerful drivers of wealth and increased well-being. However, the focus on industrial growth and technology development has increased greenhouse gas emissions and further diminished the availability of natural resources. The new technologies and practices should be used in line with two following objectives: (i) the full value of used products should be recovered for reaching a maximum of economic efficiency, and (ii) by recovering this value, the pressure on the nature should be lowered. These two objectives lead together to the rising trend of conversion from the linear economy approach to the circular economy (CE). The linear economy is based on the so called “take-make-use-disposal” approach as shown in Figure 1(a). In terms of consumers products, the waste materials after use are discarded into the environment and thereby polluting it. This is the root cause of global issues, like waste disposal in natural areas, resource scarcity, and climate change. On the other hand, CE is based on “make-use-reuse-repair-recycle” approach in a circular way as shown in Figure 1 (b). A circular economy designs out waste and pollution by reusing and repurposing products and materials that would normally be discarded into the environment. By this way CE contributes to meet the requirements of the social, economic and environmental sustainable development.
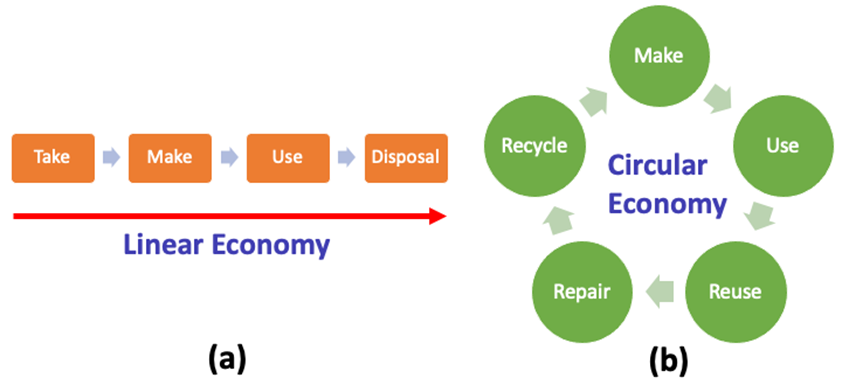
Figure 1. Schematic representation of linear economy (a) and circular economy (b) [1]
In this regard, the focus of my research activities covers two major areas of circular economy including (1) reduction of plastic waste and (2) industrial decarbonization. The objective of my investigation is to design green catalytic materials by means of a combined multiscale modelling approaches which can integrate various levels of theory.
(1) Reduction of Plastic Waste
Plastics have infiltrated every facet of human life as they are widely used in packaging, medical equipment, electronic devices, and many other fields. The current production and consumption pattern of plastics have driven a dramatic increase of plastic waste around the world. The overall global recycling rate is low; only 16% of plastics were recycled, 12% were incinerated and 66% were estimated to be landfilled or leaked to the environment in 2018. The increasing concern regarding the environmental impact of plastic waste and the plastic-related emission of greenhouse gases (GHGs) has prompted the transition towards a ‘circular plastic economy’. In a circular economy, the use of non-renewable resources and waste production is minimized, while reuse, recycling and upcycling dominate the life cycles of materials.
The collaborative research efforts between the Liverpool and Cardiff team aim to conduct elegant and detailed investigations of the synthesis, atomic structure elucidation and characterisation of new heterogeneous catalysts for the conversion of polymer plastic wastes into liquid hydrocarbons. The considered catalytic systems include a combination of metals (M= Pt, Ru, W) with metal oxides, i.e. MgO, TiO2, Al2O3, CeO2, ZnO and SrTiO3 perovskites.
In the first step of the project, we have studied the interaction of pentane (C5H12) on Pt(111) and Ru(0001) surfaces theoretically using DFT-D3 approach as implemented in VASP package. Many test models were considered by varying the distance of C atoms of C5H12 from the surface planes. According to our study the horizontal adsorption is the most stable. We have performed successive dehydrogenation (dehydrogenation of 1 H and dehydrogenation of 2 H atoms) and successive hydrogenation of pentane on the Pt(111) and Ru(0001) surfaces. The end product for successive dehydrogenation is alkene and that for successive hydrogenation is lower alkane as shown in Figure 2. In the next step, we aim to study the mechanisms of polypropylene deconstruction and upcycling (hydrogenation and dehydrogenation) on SrTiO3 support catalyst.
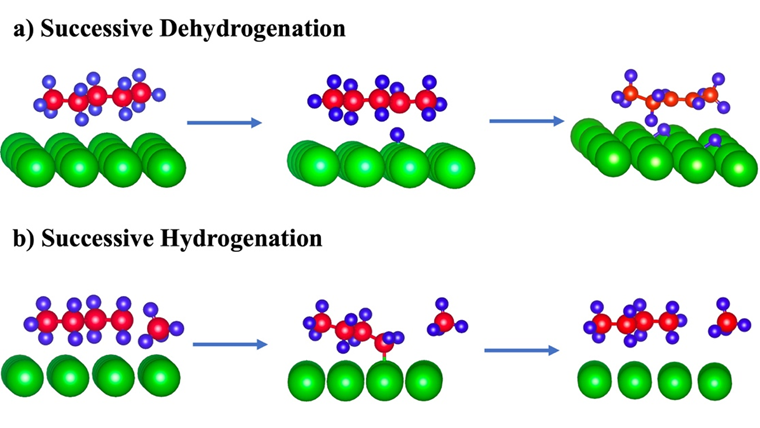
Figure 2. Catalytic dehydrogenation and hydrogenation of hydrocarbons. (a) Successive dehydrogenation showing the conversion of alkane to alkene. (b) Successive hydrogenation showing the conversion of large alkanes into lower alkanes.
(2) Industrial Decarbonization
Carbon capture and storage (CCS) are broadly recognised to play a key role in decarbonising industry, delivering low carbon heat and power, removing CO2 from the atmosphere, and, thereby, meeting global climate change targets. As a renewable and environmentally friendly source of carbon, catalytic approaches for CO2 capture, conversion, storage and utilization offer the way to mitigate the increasing CO2 build-up, and form the main focus of my interest. The objective of my investigation is to design various green catalytic materials by means of a combined multiscale modelling approaches to be utilized for chemical and electrochemical reduction of CO2.
2.1 Chemical methanation of CO2
The methanation of CO2 is the central process in the ‘Power to Gas’ (PtG) technology that links the power grid with the gas grid by converting surplus electrical energy from renewable sources into a grid compatible gas. A PtG plant basically consists of a water electrolyzer, a CO2 separation unit or source of CO2, and a methanation module as shown in Figure 3. In the methanation unit, H2 is reacted with CO2 which can be obtained from purified biogas, biomass or from the capture of an industry that has to pay a fee for its emission. H2 and CO2 are then converted into a gas mixture in the methanation unit that mainly contains CH4 and H2O. The product gas is then treated to a methane-rich gas, known as synthetic natural gas (SNG). The resulting SNG can be injected into the existing gas distribution grid or gas storages or used as compressed natural gas (CNG) motor fuel or utilised in all other well-established natural gas facilities.
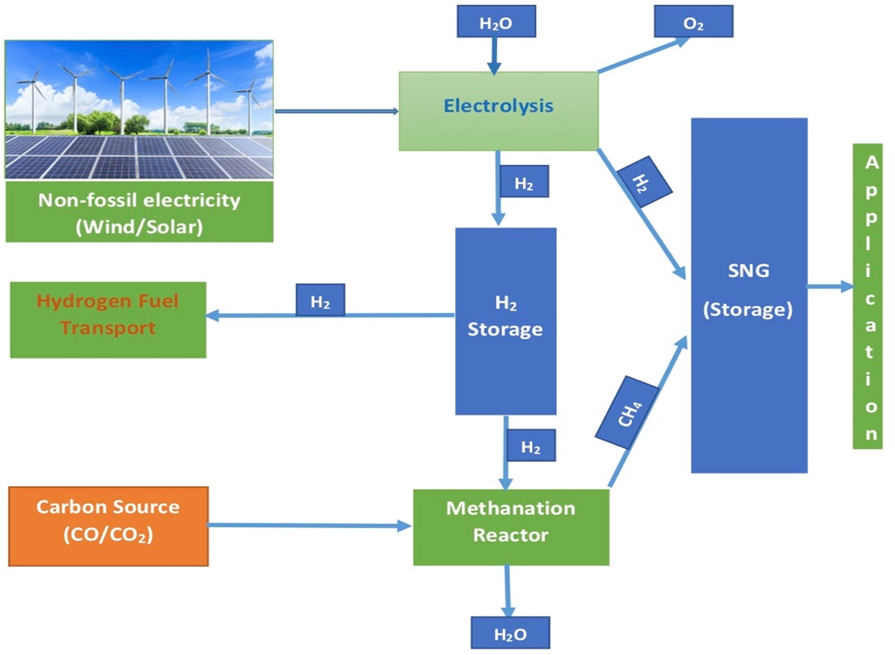
Figure 3. Schematic overview of Power to Gas (PtG) [2]
The methanation of carbon dioxide is an exothermic catalytic reaction and is typically operated at temperatures between 200°C and 800°C depending on the catalyst used. The process should be run with multiple stages to keep the temperature and consequently the concentration of carbon monoxide low. The choice of catalysts has a significant influence on this aspect. The state-of-the-art catalyst is based on nickel because of the high activity and the comparatively low price of nickel. However, there is a high risk of decreasing the longevity of catalytic materials due to less stability with fast temperature changes or catalytic poisons due to sulphur or siloxane. Therefore, considerable amount of research efforts has been given in last few years to design a new efficient catalysts that can withstand the fast changes of temperature as well as resist the catalytic poison to enhance the longevity for the methanation of CO2. One example is the catalyst material based on hydrotalcite mineral which could be a potential alternative to the traditional metal-based catalysts, and therefore the topic of my interest.
2.2 Electrochemical reduction of CO2
Electrochemical reduction of CO2 can proceed through variable number of electron reduction and proton transfer pathways in liquid- and gas-phases at both low and high temperatures. The major reduction products are carbon monoxide (CO), organic acids (formic acid and acetic acid), methane, alcohols (methanol, ethanol, and n-propanol), C2 products (ethylene and ethane) and other hydrocarbons. The major parameters that define the performance of the electrochemical technology consist of current densities, Faradaic efficiencies (FE) and overpotentials.
The gas-phase CO2 electrolysis has shown a lot of promise to be applied for the large-scale industrial application due to a drastic increase in the current densities and Faradaic efficiencies as well as reduction of overpotentials, compared to the liquid-phase technologies. Here CO2 reduction is achieved by flowing pure CO2 gas on the catalytic layer of the gas-diffusion electrodes (GDEs). The main feature of the gas-phase electrolysis cell is the type of electrode materials where a gas diffusion layer is integrated with the catalytic layer. The aim of this part is to design GDEs by different combination of materials employing both experimental and computational approaches in order to yield high current density of the electrochemical CO2 reduction. For this project, I plan to collaborate with the experimental research group of Liverpool and modelling group of Loughborough.
References:
[1] M. M. Islam, “Green Energy Development in the Era of the Fourth Industrial Revolution: Lessons from Advanced Nations” in The Bangladesh Economy(Eds. Moazzem Hossain, Qazi Kholiquzzaman Ahmad, M. M. Islam). Publisher: Routledge | Taylor & Francis Group (In Press)
[2] M. M. Islam, “Power to Gas: A Green Technology for Decarbonization of Energy Sector”, in Climate Adaptation for a Sustainable Economy (Eds. Moazzem Hossain, Qazi Kholiquzzaman Ahmad, M. M. Islam). Publisher: nova science publishers, NY, USA. ISBN: 978-1-53616-927-0 (Feb, 2020)